中国药典2020年版二氧化碳中对碳氢化合物的含量有着严格的控制指标。
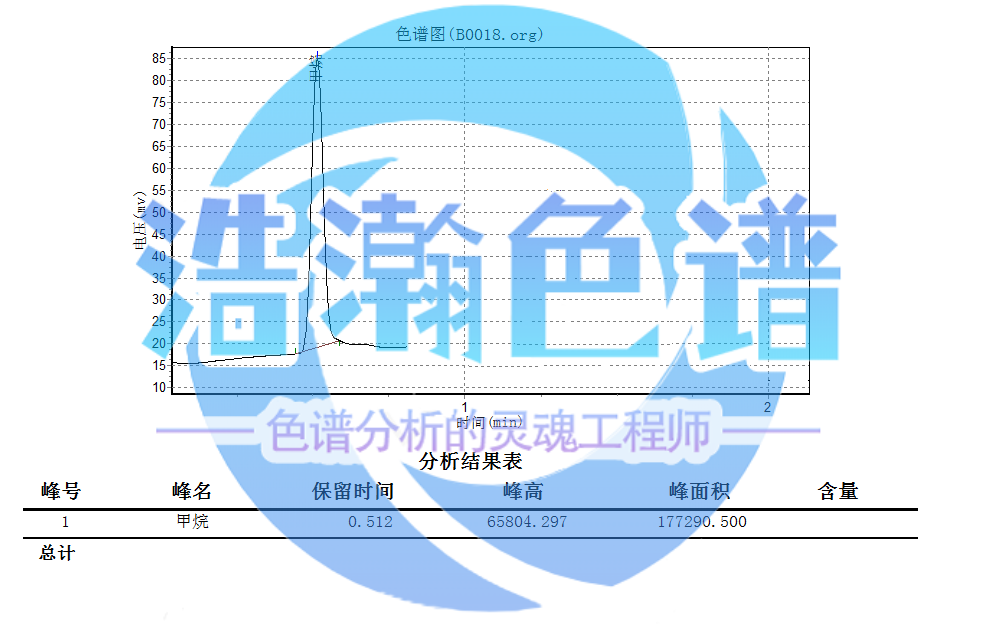
碳氢化合物照气相色谱法(通则0521)测定供试品气体取本品,即得对照品气体取甲烷含量为0.0020%的气体(以氮气为稀释剂)。色谱条件用玻璃球为填料的色谱柱(4mm×0.8m,80目);柱温为110℃;进样口温度为110℃;检测器为火焰离子化检测器,温度为120℃测定法精密量取供试品气体与对照品气体,分别注入气相色谱仪,在净化温度为360℃时测得的峰面积为相应空白值;精密量取供试品气体与对照品气体,分别注入气相色谱仪,测定峰面积,减去相应空白值后的峰面积为校正峰面积限度按外标法以校正峰面积计算,含碳氢化合物(以甲烷计)不得过0.0020%。
浩瀚色谱(山东)应用技术开发有限公司,与时俱进,在王经理的带领下,根据《中华人民共和国药典2020第四部》中二氧化碳的要求,成功的研制生产新一代HHO-III Purification device二氧化碳净化装置,在安捷伦Agilent7820A,7890B,8860,8890,岛津ShimadzuGC-2010,GC-2014等气相色谱仪上安装测试,结果满意。此款产品在制药单位安装使用,效率高,效果满意。
名称:药典二氧化碳(CO2)净化装置
型号:HHO-III Purification device
应用:2020版中国药典二氧化碳中碳氢化合物测定 气相色谱法





China Pharmacopoeia 2020 edition carbon dioxide has strict control indicators for hydrocarbon content.
Hydrocarbons shall be determined by gas chromatography (general rule 0521), and the gas of the test sample shall be taken as this sample, that is, the reference gas shall be taken as the gas with methane content of 0.0020% (nitrogen as diluent). Chromatographic conditions: chromatographic column with glass ball as packing (4mm × 0.8m, 80 mesh); The column temperature is 110 ℃; The sample inlet temperature is 110 ℃; The detector is a flame ionization detector. The temperature is 120 ℃. The determination method accurately measures the test sample gas and the control sample gas and injects them into the gas chromatograph respectively. The peak area measured at the purification temperature of 360 ℃ is the corresponding blank value; Accurately measure the test sample gas and the control sample gas, inject them into the gas chromatograph respectively, and measure the peak area. The peak area after subtracting the corresponding blank value is the limit of the corrected peak area. The external standard method is used to calculate the corrected peak area, and the hydrocarbon content (calculated by methane) shall not exceed 0.0020%.
Haohan Chromatography (Shandong) Applied Technology Development Co., Ltd., keeping pace with the times, under the leadership of Manager Wang, has successfully developed and produced a new generation of HHO-III Purification device carbon dioxide purification device according to the requirements of carbon dioxide in the Pharmacopoeia of the People's Republic of China 2020 Part IV, and installed and tested on Agilent 7820A, 7890B, 88608890, Shimadzu GC-2010, GC-2014 and other gas chromatographs with satisfactory results. This product has been installed and used in pharmaceutical companies with high efficiency and satisfactory effect, and is highly praised by users.
Name: Pharmacopoeia carbon dioxide (CO2) purification device
Model: HHO-III Purification device
Application: Determination of hydrocarbons in carbon dioxide in Chinese Pharmacopoeia 2020 by gas chromatography
滕州市浩瀚色谱仪器技术服务有限公司
电话:0632-5667636
传真:0632-5667636
手机:15562228838,13963221227
地址:山东滕州市平行路(商务部和技术部)
邮箱:wangxiaoying9@126.com
QQ:1404939462
联系人:王经理
网址:www.haohansepu.com